Sodium Alginate
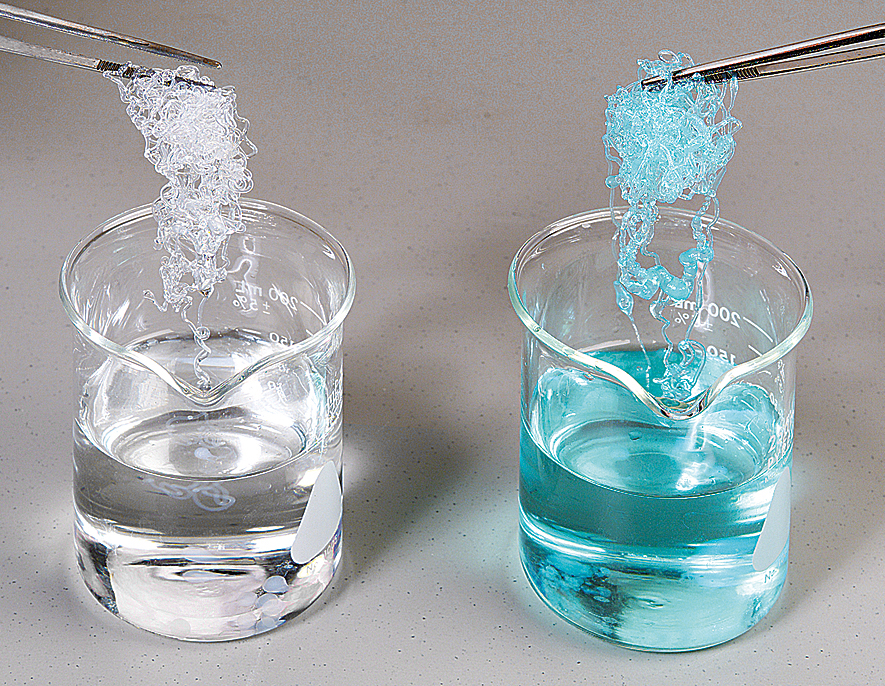
Sodium Alginate is a natural polysaccharide derived from brown seaweed or algae. It is a water-soluble compound and is commonly used in various industries, including food, pharmaceuticals, and textiles. Sodium Alginate can form gels when it comes into contact with calcium ions. This process, known as ionotropic gelation, involves the cross-linking of alginate molecules to create a gel structure. It’s commonly used in molecular gastronomy to create gel-like textures in foods like caviar or spheres.

- Overview
- Application
- Specification
Sodium Alginate is a natural polysaccharide with a diverse range of applications, derived from brown seaweed and algae. Its unique properties make it a valuable ingredient in several industries. In the realm of food, it acts as a proficient thickener, stabilizer, and gelling agent, enhancing the texture and quality of products like ice cream, dressings, and jams. In pharmaceuticals, it plays a crucial role as a binding agent in pills and tablets. Sodium Alginate is also integral in the textile industry, where it serves as a thickener for dye pastes, aiding in precise and controlled fabric coloring. Furthermore, its emulsifying and thickening qualities find utility in cosmetics and skincare products, contributing to the formulation of creams, lotions, and masks. With its ability to form gels through ionotropic gelation, Sodium Alginate has even found its place in the world of molecular gastronomy, enabling chefs to craft unique culinary textures. Recognized as safe for consumption and suitable for both vegetarians and vegans, Sodium Alginate continues to be a versatile and indispensable ingredient across various domains.
Manufacturing Process:
1. Alginic acid method Acid is added to the extract, and alginic acid is formed as gelatinous pieces. This is removed by floatation facilitated by carbon dioxide, which is obtained when excess carbonate is added initially. The remaining extract contains tiny alginic acid, 1 – 2%, which is concentrated to 7 – 8 % by centrifuging. To this alcohol, the solution is added, and then solid sodium carbonate is added till the required pH is reached. The sodium alginate is extruded in the form of pellets, oven dried and milled.
2. Calcium Alginate method This method adds calcium chloride to the extract to obtain solid calcium alginate as fibres. The fibrous material can be removed by sieving, and excess calcium is removed using water. It is stirred with dilute acid to give alginic acid, and this is screw pressed to form a paste. To this solid, sodium carbonate is added till the required pH is reached. The sodium alginate is extruded in the form of pellets, oven dried and milled.
Food Industry
Sodium alginate is used in the food industry to increase viscosity and as an emulsifier. Sodium alginate is used as a stabilizer for ice cream, yoghurt, butter and cheese. Sodium alginate can also be used as a thickener and emulsifier for salads, puddings, jams, tomato juice and canned products. It is used as a hydrating agent for noodles, bread, and frozen products. Sodium alginate is widely used to produce gelatinous products of sauce.
Pharmaceutical Industry
Alginate swells in water, and the pharmaceutical industry uses this property as a disintegrant. It is used in liquid medicines to increase viscosity and solid suspension. It is also used to fix cells and enzymes.
Other Industry
Sodium alginate has the ability to thicken in water, making it suitable for textile printing. It is used for the surface sizing of paper. Its oil resistance to paper is also better. Alginates are widely used in the dental industry as impression materials. It is a good chelating agent for extracting radioactive substances from the body.
Here’s a table outlining the general specifications of Sodium Alginate:
| Property | Specification |
|---|---|
| Chemical Formula | (C6H7NaO6)n |
| Appearance | White to yellowish-brown powder |
| Solubility | Soluble in water |
| Molecular Weight | Variable, depending on chain length |
| CAS Number | 9005-38-3 |
| E Number (EU) | E401 |
| Odor and Taste | Odorless and tasteless |
| pH Range | Typically between 6.0 and 8.0 |
| Particle Size | Typically fine powder |
| Density | Approx. 1.3 g/cm³ |
| Viscosity (1% Solution) | Varies depending on type and quality |
| Gel Formation | Requires calcium ions (Ca2+) for gelling |
| Shelf Life | Can vary; store in a cool, dry place |